Scientists discover “brain hot spot” for drugs against fear
Findings could lead to a new therapeutic approach
The ways in which psychiatric drugs work on the level of neural networks are not yet fully understood. A team of scientists led by Dr. Wulf Haubensak at the Research Institute of Molecular Pathology (IMP) in Vienna and Prof. Dr. Andreas Hess of the Institute of Experimental and Clinical Pharmacology and Toxicology, have now identified a neuronal circuit in the brain that plays an important role in anxiety and demonstrated how common psychiatric medication acts on it. The study has now been published in the journal “Molecular Psychiatry”.
Anxiety disorders are a major medical problem affecting a large section of the population. These disorders can be treated with a range of psychiatric drugs, including a group of substances called benzodiazepines (BZD). BZD have been used to treat patients with anxiety for 50 years and what they do on the molecular and cellular level is well understood. However, doctors and neuroscientists still know little about the neural circuit interactions through which BZD unfold their anxiety-relieving effect.
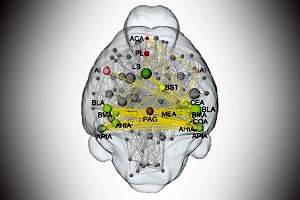
A team of scientists, led by Dr. Haubensak of the IMP and Prof. Hess of the Institute of Experimental and Clinical Pharmacology and Toxicology has now used a combination of innovative methods linking genetics, information on neuronal circuits and functional brain mapping. They found that BZD interfere with the relay of aversive signals through the amygdala and characterized the circuits involved.
“Fear emerges from the interaction of several circuits across the brain. In this network, we identified a crucial biomedical ‘hot spot’ that provides the starting point for fear-relieving therapy”, says Dr. Haubensak. “Tracking down this hot spot has only become possible by combining insights on the connections of neurons in the brain, the connectome, with genetic techniques that allow the functional visualization and manipulation of specific populations of neurons in living animals – methods and information that have become available only very recently.”
The scientists used mice for their experiments, but by comparing their findings to the functional brain scans of humans, they found clues that the same mechanisms are present in people. This opens new opportunities for drug development.
Prof. Hess, co-author of the study, emphasized the importance of functional brain imaging. “Non-invasive imaging techniques such as magnetic resonance imaging are key to studying neurobiological functions at the whole brain level. We combined this with novel data analysis strategies to identify the modulatory impact of small neuronal circuits that constitutes an important brain function – in this case anxiety.” “Since we know the exact networks of neurons that mediate the BZD anxiolytic effect, we can now try to target them specifically. This may allow the development of new drugs that treat anxiety without the side effects common to current anxiolytics”, says Johannes Griessner, doctoral candidate at IMP and first author of the study. He concludes with a broader outlook on how the findings could be used in further studies: “Psychiatry needs a strong biological basis which allows for targeted therapeutic interventions. Our approach could serve as a blueprint for an experimental strategy that could be used to better characterize the effects of psychoactive drugs in general.”
Further information
Prof. Dr. Andreas Hess
Phone: +49 9131 8522003